Jamella australiae Pandanus Leafhopper Fact Sheet
Introduction
Jamella australiae Pandanus Leaf Hopper is a pest of the native Pandanus Pandanus tectorius var. pedunculatus (Smith and Smith1 2000). Pandanus Pandanus tectorius var. pedunculatus is a dioecious tree common on the east coast of Australia north from Port Macquarie in NSW (Australian Native Plant Society 2017); [see Figure 1]. The fruit is an edible drupe (NSW Flora Online 2017). The Pandanus Leaf Hopper was first described by Kikaldy in 1906 (Medler 1990). It is in the order Hemiptera, family Flatidae (Atlas of Living Australia 2017). It is one of two Australian native species of Jamella. It is endemic to and was described in Cairns and Townsville (Medler 1990). In north Queensland the native wasp Aphanomerus pusillus parasitises the eggs (Smith and Smith1 2000) and controls the disease.
The pest appears to have moved from the initial infestation point on the Sunshine Coast by the insects jumping or flying from tree to tree and also from people moving infected trees (Smith and Smith1 2000).

Figure 1.
Pandanus tectorius trees in Byron Bay.
Biology
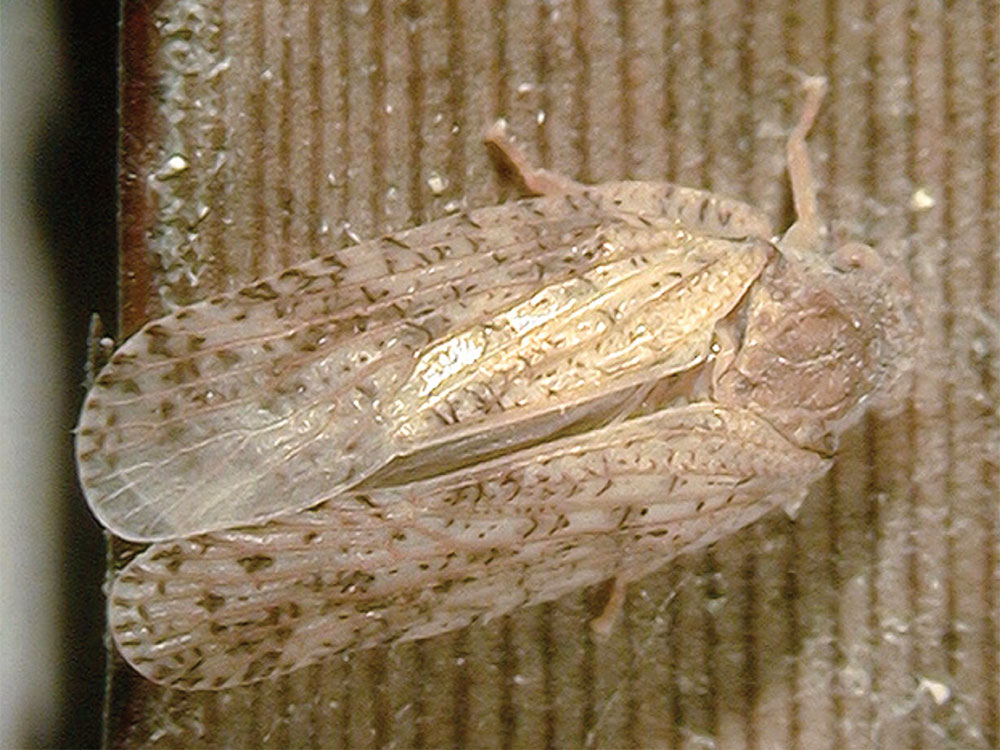
The insect is a sap sucker. The nymphs produce a distinctive white covering and are white with a fluffy rear (Brideson undated). The nymphs [see Figure 2] have five instar stages ranging from 0.32 to 1.22 mm wide heads and the life cycle can take as little as eight weeks giving 4-5 generations per year in southeast Queensland (Smith and Smith1 2000). The adult is winged and is usually about 8 mm in length [see Figure 3]. They can fly but prefer to jump hence their common name (Smith and Smith1 2000). The adults lay their eggs in a raft of up to 80 eggs on the underside of the leaves.

Figure 2.
Jamella australiae nymph.
In the early 1990’s Pandanus trees were observed to be declining and dying on the Sunshine Coast. Pandanus Leaf Hopper was identified as the pest causing the disease (Smith and Smith1 2000). Pandanus trees in Broadbeach and Miami on the Gold Coast were also observed to be affected by Jamella australiae and dying. In the early 2000’s pandanus trees in northern NSW were also noticed to be affected in 2004 (Brideson undated). Pandanus trees were also observed to be affected by Leaf Hopper in Happy Valley, Fraser Island in 2011 (Sinclair 2011).
The temperature affects the viability of the flatid. In studies carried out by Smith and Smith (2000) it was shown that nymphs died and eggs didn’t develop at constant temperatures above 320C and below 150C. The flatid was able to complete its life cycle at temperatures between 20 and 290C.
The disease can be confused with infestation by Mealy Bugs Pseudococcus sp. [see Figure 4] that are also a sap sucking insect (Smith N 1998). Like J. australiae they also
produce a black sooty mould on the leaves and trunk of the tree, encourage fungal pathogens and cause decline and death of the growing tips and eventually the whole tree (Thomson et al. 2006).

Figure 3.
Adult Jamella australiae.
From NSW Dept. Ag. Biosecurity Collections.

Figure 4.
Mealy bug nymph found on Pandanus leaf.
Ecology
The Leaf Hopper has not been recorded further south than Yamba (Brideson undated) and it would appear that temperature is a limiting factor in the range of the insect. The Planthopper nymph feeds on the leaves particularly where they are closely packed at the base [see Figure 5]. They feed on the sap of the tree by sucking it from the phloem in the leaves (Wilson and Lucchi 2006).
They cannot utilise all the plant sugars and exude a honeydew that is colonised by mould fungi causing a black sooty covering of the leaves and trunks. When they occur in high numbers the damage to the leaves causes them to become vulnerable to secondary fungal infections [see Figure 6]. Smith and Smith1 (2000) found a high correlation between the numbers of flatids and tree health. Where numbers are high decline and death of the growing tips can occur eventually lead to death of the tree (Brideson undated).
The adults can jump and fly and the life cycle is also short so the insects can infect all the trees in an area in a relatively short time. In the late 1990’s half or more of the Pandanus population in Noosa and Burleigh Heads were found to be heavily infected or dead (Smith and Smith1 2000).
A survey was carried out in the Ballina Shire in NSW in 2006 that found approximately 14% of the surveyed trees were affected (Brideson undated).

Figure 5.
The signs of infestation by Planthopper are the white fluffy material and close inspection shows the nymphs and egg rafts.
Photo by L. Behrendorff Queensland Parks & Wildlife Service

Figure 6.
Secondary infection by fungal disease causes death of the leaves and growing tips.
Management
Pandanus Leafhopper caused the death of a large number of Pandanus trees on the Sunshine Coast when it first established, on the north coast of NSW and Fraser Island (Brideson undated; Sinclair 2011). By 1997 up to 75% of Pandanus trees were either dead or dying in Noosa (Smith and Smith1 000).
Trials were undertake to test the efficacy of a number of chemical pesticides. Three chemicals; imidacloprid, monocrotophos and dimethoate were injected into the trunk of infested Pandanus trees and also spot sprayed onto the growing heads of the trees (Smith and Smith2 2000). All three chemicals gave good results. After 3 months the trees treated by injecting the trunks with monocrotophos and dimethoate were re- infested but the trees treated with imidacloprid remained free of the flatids. The effect of the chemicals was slow with injections, the fastest some control was achieved was 8 days with imidacloprid. There was a significant delay in the results for trees that were badly infested and were in poor condition. It can be assumed that this was due to the poor translocation rate in the growing heads that were in poor condition. Control with imidacloprid was still evident after 2 years. The drill holes used to inject the trees showed dead areas around the drill holes with imidicloprid having the smallest amount of necrosis. The smallest amount of necrosis occurred when using drill holes of 6 mm. On large trees it was observed that injecting the trees as high up on the branch as possible gave the fastest results. It was shown to be important to choose branches that were large enough to withstand the drill holes and resulting necrosis.
The tests using spot spraying of the three chemicals also showed control by all three. The most cost effective dose rate for imidacloprid was 0.5 ml per 100 ml of water applied to each head. The trees sprayed with imidacloprid showed no infestation after 12 months. Since monocrotophos and dimethoate are fairly toxic to animals it was determined that spot spraying with these chemicals, particularly in public spaces is not acceptable.
While the area of damage around the drill holes using 6 mm holes injected with imidacloprid was approximately 5 mm and the structural integrity of the tree was not compromised, repeated drilling is likely to have a detrimental effect. Spot spraying does not damage the tree but is problematic on large trees and for trees in public spaces.
Some 3,000 trees were treated by injecting with imidacloprid on the Sunshine and Gold Coast in 1997. Good results were obtained with twice the number of trees in good condition after 12 months (Smith and Smith2 2000). Several hundred trees were also injected with imidacloprid on the north coast of NSW in 2006 with young trees spot sprayed (Walker and Wellman 2005).
In addition to spraying with insecticide, removal of dead and dying leaves was also shown to be effective in long term control of the flatid (Livingston Shire Council 2017).
Natural predators of the Planthopper in south east Queensland are syrphid larvae (Allobacca sp.), lacewing larvae (Oligochrysa lutea) and huntsman spiders (Smith and Smith1 2000).
In north Queensland two parasitic wasps were found to be parasitising the Planthopper eggs.
The most common was Aphanomerus pusillus with Ooencyrtus sp also being found but in much lower numbers. Both species were released in south east Queensland in 1996 with limited results. Further larger releases in 1997 did result in the establishment of populations of A. pusillus. By 1998 flatid populations had begun to drop and although the parasitoid numbers drop in winter, they are still present and are controlling the Planthopper [see Figure 7].

Figure 7.
Planthopper egg raft parasitised by A. pusillus. The holes in the eggs are the emergent holes of the wasps.
Innorthern NSWinvestigationswereundertaken to explore the potential to collect the parasitic wasp from south east QLD and release them in NSW. A licence from the National Parks and Wildlife is required however and that takes some time. In 2006 the parasitoid was found in Kingscliff so the process of obtaining a licence for its release was abandoned (Brideson undated). Currently the Planthopper has become rare on the NSW north coast and any egg rafts that are observed are invariably parasitised (pers obs. 2017).
It appears that the major threat to Pandanus from Jamella australiae has diminished with the establishment of Aphanomerus pusillus. Good cultural practices should be maintained however. They include sourcing planting material locally, checking for infestation in new planting material and removing and destroying dead and dying leaves infested with Planthopper.
References
Atlas of Living Australia. Pandanus tectorius.
Accessed 11/12/2017 https://bie.ala.org.au/species/http://id.biodiversity.org.au/node/apni/2891749
Australian Native Plant Society. Pandanus tectorius.
Accessed 11/12/2017 http://anpsa.org.au/p-tec.html.
Brideson J. undated. Protecting Pandanus Trees From the Pandanus Planthopper. Pandanus Planthopper Working Group. Ballina Shire Council.
Livingston Shire Council. Pandanus Dieback.
Accessed 11/12/2017 www.livingstone.qld.gov.au/DocumentCenter/Home/View/8609
Medler J. 1990. Review of ‘Jamella australiae’ and ‘Malleja’, gen. nov. in Australia and New Guinea, with Descriptions of New Species (Homoptera:Flatidae). Invertebrate Taxon, Vol. 3 pp 995-1004.
New South Wales Flora Online. ‘Pandanus tectorius’ Parkinson ex Du Roi
Accessed 5/11/2017
http://plantnet.rbgsyd.nsw.gov.au/cgi-bin/NSWfl.pl?page=nswfl&lvl=sp&name=Pandanus~tectorius.
Sinclair J. 2011. What has World Heritage meant for Fraser Island? Fraser Island Defenders Organisation. FIDO Backgrounder No. 52.
Smith N. 1998. Pandanus Dieback Report of the Management of Jamella australiae in Pandanus in Southeast Queensland. Queensland Department of Environment and Heritage and Queensland Department of Primary Industries.
Smith N. Smith D. 2000. Studies on the flatid ‘Jamella australiae’ Kikaldy causing dieback in ‘Pandanus tectorius var. pedunculatus’ (A.BR.) Domin on the Sunshine and Gold Coast in Southeast Queensland. The Journal of the Entomological Society of New South Wales, Vol. 29 pp 11-20.
Smith N. Smith D.2 2000. Systemic Insecticidal Control of the Flatid Jamella australiae Kirkaldy, a Pest on Pandanus in Southeast Queensland. The Journal of the Entomological Society of New South Wales, Vol. 29 pp 21-25
Thomson L. Engleberger L. Guarino L. Thaman R. Elevitch C. 2006. Pandanus tectorius. Species Profiles for Pacific Island Agroforestry, Permanent Agriculture Resources. Honlulu.
Walker L. Wellman L. 2005. Protecting a coastal icon: a cooperative approach to controlling Pandanus dieback in northern New South Wales. Australasian Plant Conservation: Journal of the Australian Network for Plant Conservation, Vol 14 No 3 pp 20 -23.
Wilson S. Lucchi A. 2006. Feeding Activity of the Flatid Planthopper Metcalfalf pruinosa. Journal of the Kansas Entomological Society. Vol. 60. Issue 1. pp 60-61.